To accompany the introduction of our llama antibody service we will be running this llama antibody blog.The last blog gave a quick introduction to llama antibodies, mainly:
- The antigen binding fragment of Llama antibodies consist of a single variable (VH) domain on a heavy chain (VHH)
- The heavy chain llama antibodies lack a light chain yet they are still fully capable of antigen binding
This time we look at the structure of llama antibodies in more detail.
In conventional antibodies, both heavy and light chain form the antigen binding site with amino acids from the second framework region (FR2) interacting with the variable domain of the light chain (VL). (The FR2 lies between the CDR1 and CDR2 regions).
Compared with conventional VH, llama VHHs differ by four amino acids in the FR2; positions (37, 44, 45 and 47; shown in green). In the conventional VHs these FR2 amino acids were conserved during evolution and are involved in forming the hydrophobic interface with the VL.
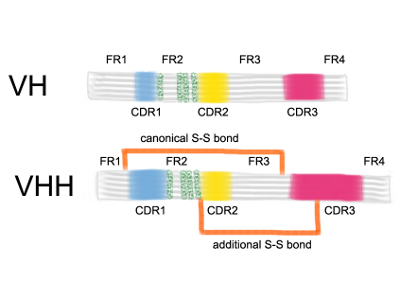
Fig: Adapted from From Wesolowski et al. Med. Microbiol. Immunol (2009) 198:157-174
The three complementarity determining regions (CDR1, CDR2 and CDR3) form the antigen-binding paratope. CDR1 and CDR2 have canonical structures and are found in the variable (V) region, whereas CDR3, which has a non-canonical conformation, includes some of the V, all of diversity (D, heavy chain only) and joining (J), and some of the constant (C) regions. The CDR3 in llama antibodies is more variable and longer than in conventional antibodies.
The lack of a light chain and the associated amino acid substitutions in the CDRs does not limit or reduce the diversity of the epitopes or antigen binding. In actual fact the amino acid substitutions and the longer CDR3 might compensate for the lack of the three CDRs carried by VL by increasing the surface size of the paratope. Furthermore, the llama CDR3 is extraordinary as it forms a convex loop extension which can extend into the active sites of antigens (e.g. enzyme active sites). This is in contrast to the flat interaction surface between Fab (VH-VL) of conventional antibody and antigen.
Llama VHH – stable and soluble
Compared with conventional antibodies, where the interface between VL and VH (for the formation of the binding cleft) is stabilized by hydrophobic residues in the FR2, these residues are hydrophilic and charged in llama VHHs making the VHHs more soluble. The extra-long CDR3 also favors the solubility of VHHs.
VHH – thermally stable
Due to the increased hydrophilicity, llama VHHs are conformationally and thermally more stable. Unlike conventional antibodies they are functional at high temperatures (heat shock; 2 hours at 90oC) and refold after heat denaturation. Refolding of llama antibodies is induced after contact interaction between the CDR3 in the unfolded VHH with antigen.
Biotechnological and therapeutic application
Immunomodulation
Where conventional antibodies fail to recognize less antigenic sites, VHH can be engineered. Recombinant VHH can be expressed at high levels in prokaryotic (Escherichia coli) and eukaryotic cells (yeast). VHH antibody fragments have been shown to be potent enzyme inhibitors in vitro. The use of VHH to inhibit enzyme activity to bring about immunomodulation is more efficient than using antisense technology.
VHH humanized antibodies are being used to target tumors:
- Treatment of solid tumors where VHHs binds to the epidermal growth factor receptor so blocking the binding of epidermal growth factor (ECF).
- Treatment of rheumatoid arthritis where VHHs binds to tumor necrosis factor- alpha (TNFa)
- Targeting drugs across the blood -brain barrier for use in neurodegenerative diseases.
Penetrating tissues where conventional antibodies can’t reach
The advantage of VHHs being small allows them to pass across the blood brain barrier with ease, making them an ideal imaging tool for targeting cryptic antigens. Llama VHHs selected by phage display against tumor markers (e.g. EGFRIGF-1R) are being used successfully in cell imaging studies, anti-cancer therapy, and in vivo imaging of tumor tissue.
Llama antibodies as neutralizing antibodies
Llama antibodies have been used against rotavirus in vivo and used to identify caries caused by streptococcus mutans. As VHHs are small and soluble they are rapidly cleared from the kidney. Alternatively, bispecific VHHs can be generated which recognize long-lived serum proteins (e.g. Albumin, PEG), to increase half-life, and the therapeutic target of interest.
More targets, more opportunities and economically produced
The unique features of VHHs: small, soluble, fully active antibody fragments, high affinity, stable, easy to clone and express in both eukaryotic and prokaryotic systems, make them the ideal tool for research, therapy (e.g. blocking receptor interactions to treat cancers, inflammatory diseases, parasitic infections and viral infections), diagnostics (cancer diagnostic tests e.g. detecting different isoforms of PSA in the blood; in vivo imaging of tumors) and biotechnology (anti-toxin production).
Further reading:
- Desmyter A, Transue TR, Ghahroudi MA, Thi MH, Poortmans F, Hamers R, Muyldermans S, Wyns L. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat Struct Biol. 1996 Sep;3(9):803-11. PubMed PMID: 8784355.
- Chothia C, Lesk AM. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987 Aug 20;196(4):901-17. PubMed PMID: 3681981.
- Muyldermans S. Single domain camel antibodies: current status. J Biotechnol. 2001 Jun;74(4):277-302. PubMed PMID: 11526908.
- Wesolowski J, Alzogaray V, Reyelt J, Unger M, Juarez K, Urrutia M, Cauerhff A, Danquah W, Rissiek B, Scheuplein F, Schwarz N, Adriouch S, Boyer O, Seman M, Licea A, Serreze DV, Goldbaum FA, Haag F, Koch-Nolte F. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med Microbiol Immunol. 2009 Aug;198(3):157-74. PubMed PMID: 19529959.
- Lauwereys M, Arbabi Ghahroudi M, Desmyter A, Kinne J, Hölzer W, De Genst E, Wyns L, Muyldermans S. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 1998 Jul 1;17(13):3512-20. PubMed PMID: 9649422.
- Schwall GP, Safford R, Westcott RJ, Jeffcoat R, Tayal A, Shi YC, Gidley MJ, Jobling SA. Production of very-high-amylose potato starch by inhibition of SBE A and B. Nat Biotechnol. 2000 May;18(5):551-4. PubMed PMID: 10802625.


